En iyi web sitesi deneyimi için lütfen konumunuzu ve dilinizi seçin

Lately, there’s been quite the buzz around the European Union Medical Device Regulation or better known as, EU MDR. The EU MDR is a priority for all companies in the medical device industry that distribute products in the EU and will officially take effect on May 26, 2021. This regulation has more need for data management and complex assessment procedures than the regulation it replaces, the Medical Devices Directive (MDD). All other classes of devices can continue to be sold under a valid MDD certificate until May 2024 or the MDD certificate’s expiry date. As the deadline approaches, there are some key labeling elements that medical device companies must adhere to for EU MDR compliance. Within this blog post, we will break down what EU MDR is and how it will impact your labeling process.
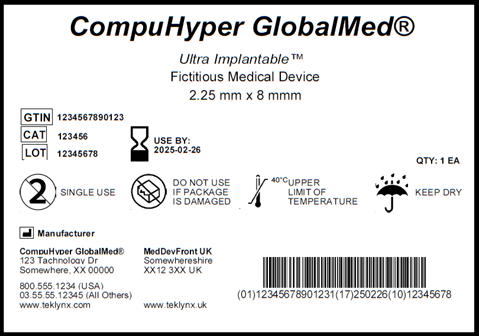
The EU MDR is designed to ensure public health and patient safety across Europe and to increase quality and transparency of medical devices in the healthcare supply chain through label design, label approval, and label tracking standardization. Many organizations within the healthcare industry who have medical devices that are implantable, life-supporting, or life-sustaining must also bear a Unique Device Identifier (UDI) on its labels, which includes:
UDI issuing agencies for EU MDR are:
All medical device manufacturers doing business in Europe had to comply with EU MDR guidelines by May 26, 2021. This regulation applies to all organizations doing business in Europe, meaning medical devices manufactured outside of the EU, but distributed throughout European Union nations will also need to comply. The final compliance deadline for all classes of medical devices manufactured is May 26th, 2024.


Whenever a new regulation is introduced, it can be stressful and overwhelming to iron out the new guidelines that you must comply with. With new designs, icons, and even sizes of the labels, it can be difficult to find EU MDR labeling software for your company.
The cost of not having EU MDR labeling software implemented for your company can cause even greater stressors for your company though by facing quarantined products, recalls, and even financial penalties or other sanctions due to non–compliant labels. Luckily for you, TEKLYNX has two EU MDR labeling software solutions that will help you easily and seamlessly comply with this regulation.
DOWNLOAD FREE TRIAL OF CODESOFT
Don’t wait to start using EU MDR labeling software!
REQUEST A DEMO OF TEKLYNX CENTRAL CFR
Doug Niemeyer is the President & General Manager at TEKLYNX Americas. He leverages his 25+ years of sales & marketing experience in technology and leadership to help grow the business. His passion for leadership, insatiable curiosity, and competitive spirit help to drive success, encourage progress and development while ensuring teams hold positive customer experiences in the highest regard, all with the purpose of helping companies barcode better. When he is not working you can find him in a gym, on a field or golf course playing or coaching.
In today’s day and age, you can track just about anything; when your Amazon package will be delivered, where your friends and family are located, and even your pizza delivery! But what about your barcode labels?
READ MORE
Imagine painting a room and gathering supplies. You wouldn’t want to stop at three separate stores for your paint, primer, and brushes. It’s more convenient and efficient to stop at a store that carries everything you need, right?
READ MORE
When it comes to your barcode labeling software solution, implementing lean labeling and continuous improvement principles to your labeling environment reduces waste in label printing and saves you money.
© Telif hakkı 2025 TEKLYNX CORPORATION SAS. Tüm Hakları Saklıdır.
What do you think? Leave us a comment.
Comments will be reviewed and are subject to TEKLYNX’ comment policy. Your email address will not be published publicly.