Seleccione el idioma de su ubicación para obtener la mejor experiencia del sitio web
Seleccione el idioma de su ubicación para obtener la mejor experiencia del sitio web

If you encountered a labeling error today, would you be able to confidently pinpoint how and why it took place? Unfortunately, for many companies, the answer is “no.”
Many companies have a label design and approval process that is informal, manual, and prone to mistakes. Labeling mistakes are no small issue: at least 40% of recalls published by the United States Food and Drug Administration (FDA) are due to labeling errors. For example, Van Law Food Products Inc. recently recalled Whole Foods Market 365 Organic Creamy Caesar Dressing because it contained undeclared soy and wheat allergens. Product recalls can be extremely costly, from wasted products to additional labor costs; not to mention the damage to the brand from negative press.
So, how can you reduce the risk of labeling errors to near zero? You can set your company up for success by implementing an automated label audit trail. A label audit trail is a reliable, consistent way of documenting changes made to label designs. Maintaining an accurate label audit trail reduces the risk of labeling errors and helps pinpoint the cause if they happen.
Audits are most associated with the accounting and tax world, but did you know audits are used by a variety of industries for a wide range of purposes? An audit trail is simply a record of changes made to an entity. When it comes to labeling, a label audit trail is an accurate and detailed record of changes made to labels and actions taken with those labels. A detailed label audit trail contains data such as:
Because the label audit trail includes records of any changes made to the label design, it also supports a label version history. Using label version history, you can review the changes made over time and even revert to a previous version if necessary.
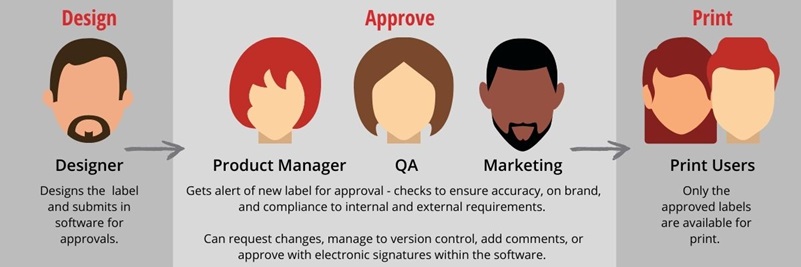
For example, a label audit trail could tell you that Darryl requested a new label template for a new line of pre-made salads. You could then see that Judith created the first draft of the label and sent it to Sam for review. Sam sent back edit requests to include proper allergen labeling for the wheat in the croutons and almonds in the crunchy topping. Judith made the edits and Sam approved the label. You could see that Kelly printed a batch of 50 labels with an expiration date of November 1, 2022, on your Zebra ZT400 label printer. One day, the allergen statement goes missing from your salad labels! Luckily, Kelly notices before the salads are labeled. By looking at your label audit trail, you see that your new employee, Brent, made changes to the label, and Sam approved it without any edit requests. Now you can provide further training for Brent and Sam to prevent the mistake from happening again.
Having a detailed label audit trail has numerous benefits, from improving internal processes to minimizing damage in the event of a labeling-related recall. Here are three of the most important benefits:
Reducing labeling errors has a ripple effect across the business, all the way to the bottom line. When fewer labeling errors are made, fewer products, labels, and boxes are wasted. Employees spend less time correcting and reprinting labels, allowing them to focus on value-added activities. Overall productivity and efficiency increase while waste and rework decrease. The result? A better bottom line.
Maintaining a label audit trail is required in certain industries. The FDA 21 Code of Federal Regulations (CFR) Part 11 labeling regulation requires medical device manufacturers and pharmaceutical companies to maintain records of their labeling process. Electronic signatures, a feature of labeling audit trail software, are required for accountability and security.
For the food and beverage industry, while label audit trails are not required by any federal regulation, labeling accuracy is crucial. Other regulations and initiatives such as the Food Safety Modernization Act (FSMA), Food Allergen Labeling and Consumer Protection Act (FALCPA), and Produce Traceability Initiative (PTI) are in place to promote the safety of the food supply chain. Food and beverage companies can maximize their protection against labeling errors by taking advantage of a label audit trail.
Other industries, such as chemical, aerospace & defense, and cannabis, have similar safety labeling requirements. While a label audit trail is not strictly required, the benefits of having one in place are highly valued by companies in these industries.
Whether your company operates in a highly regulated industry or not, ask yourself: Would a labeling error ruin my day? If the answer is yes, consider implementing a label audit trail to quickly identify and fix labeling errors before they have a big impact on production.
To set up a label audit trail and protect your company from labeling errors, follow these recommendations:
Want to see how LABEL ARCHIVE automatically creates a label audit trail?
Now that you know what a label audit trail is and why it’s so important, ask yourself this question: Can my company afford not to have a label audit trail in place?
TEKLYNX has years of experience implementing label traceability systems for thousands of customers. Get in touch with our labeling experts to find out how we can help you achieve your labeling goals.
Casey Sciano is an Enterprise Executive at TEKLYNX. He uses his 15+ years of sales and technical experience and knowledge of the AIDC space to support enterprise label management clients. Casey strives to help clients "see the forest through the trees" by utilizing sound business strategies and sales techniques that impact the way they do business. When not working, he loves spending time with his dog Josie and cheering on the Brewers, Bucks, and Packers.
One of manufacturing's biggest fears is a product recall - not only because of the potential for bad PR, but because of the wasted product, money, time, and potential safety risks for the users of their products.
READ MORE
A common labeling challenge I’ve been hearing recently is the need to move toward a paperless label approval process. Instead of printing out sample labels, physically delivering them to each person in the approval process, and returning to the label design software to implement changes, companies are looking to digitally transform this process and make label approval paperless.
READ MORE
Este blog trata de las normas de etiquetado de seguridad alimentaria, la preparación de la Sección 204 de la FSMA de la FDA, la importancia de la precisión del etiquetado para evitar las retiradas, y si una retirada es inevitable, cómo ayudan las soluciones de etiquetado a identificar los productos que hay que retirar de la cadena de suministro.
SABER MÁS© Derechos de autor 2025 TEKLYNX CORPORATION SAS. Todos los derechos reservados.
What do you think? Leave us a comment.
Comments will be reviewed and are subject to TEKLYNX’ comment policy. Your email address will not be published publicly.